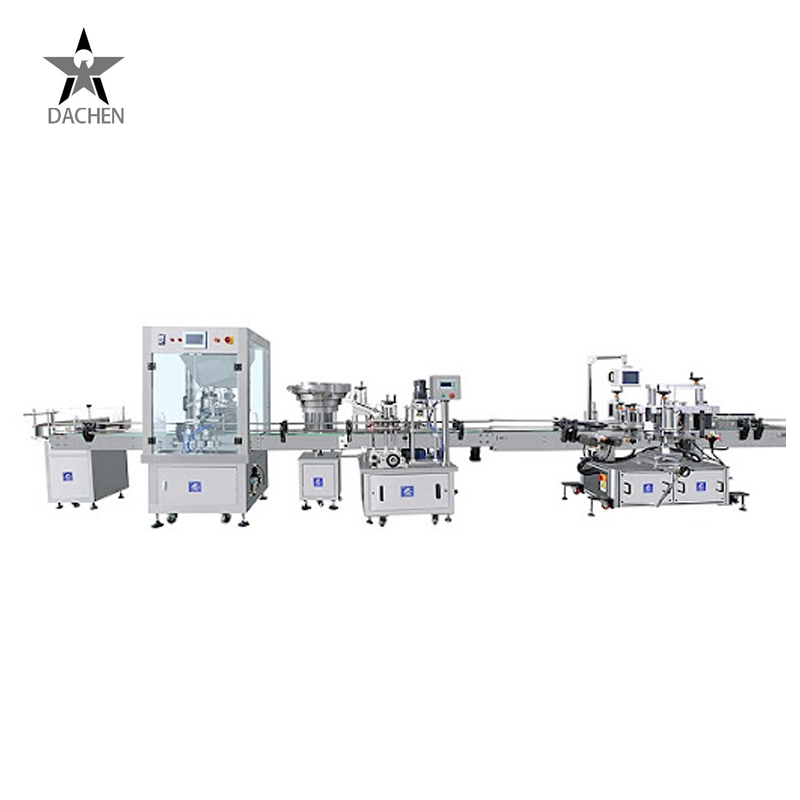
Modular and Customizable Design
Configurable based on the type of medicine (solid, liquid, or injectable) and the packaging format.
Easy integration with upstream and downstream equipment for seamless production.
High-Speed Packaging
Capable of handling high production volumes with consistent accuracy.
Supports various packaging formats, including blister packs, strip packs, bottle filling, and carton packaging.
Precision Filling and Sealing
Utilizes cutting-edge technologies such as:
Blister Forming and Sealing: Thermoforming or cold-forming for blister packs.
Liquid Filling: High-precision filling nozzles for syrups, suspensions, or injectable solutions.
Powder Filling: Auger-based or volumetric fillers for powdered medications.
Advanced Inspection Systems
Inline systems for quality control, including:
Vision systems for detecting missing tablets/capsules or misaligned packaging.
Leak detection for blister packs.
Weight verification for bottles or sachets.
Smart Control Systems
Equipped with programmable logic controllers (PLCs) and human-machine interfaces (HMIs) for centralized control.
Real-time monitoring of production parameters, including speed, temperature, and quality metrics.
Automated error detection and alarm systems for minimal downtime.
Versatile Labeling and Printing
High-speed labeling for bottles, cartons, or blister packs.
Integration of printing systems (e.g., inkjet or laser printers) for batch numbers, expiration dates, and barcodes.
Compliance with Pharmaceutical Standards
Fully compliant with GMP (Good Manufacturing Practices), FDA, and ISO standards.
Designed with stainless steel and pharmaceutical-grade materials to ensure hygiene and safety.
Efficient End-of-Line Automation
Automatic cartoning, case packing, and palletizing.
Integration with serialization and track-and-trace systems to meet regulatory requirements.
Process Workflow
Product Feeding
Automatic feeding of tablets, capsules, liquids, or powders into the packaging line.
Sorting and orientation systems ensure proper alignment.
Primary Packaging
Blister Packaging: Medicines are placed into pre-formed cavities, sealed with lidding material (aluminum or film).
Bottle Filling: Bottles are automatically filled with tablets, capsules, or liquids, followed by capping and sealing.
Sachet Packaging: Powders or liquids are filled into individual sachets and sealed.
Quality Control
Inline vision inspection checks for missing products, damaged packaging, and correct alignment.
Leak detection systems for ensuring the integrity of sealed packs.
Secondary Packaging
Packaged medicines are placed into cartons, labeled, and sealed.
Serialization and track-and-trace codes are applied for regulatory compliance.
End-of-Line Automation
Cartons are grouped, shrink-wrapped, and packed into shipping boxes.
Palletizing systems arrange boxes for easy transport and storage.
Applications
This production line is suitable for packaging:
Tablets and Capsules: Blister packs, bottles, or strip packs.
Liquid Medicines: Syrups, suspensions, or ampoules.
Powdered Medicines: Sachets, bottles, or pouches.
Injectable Solutions: Pre-filled syringes or vials.yy
Benefits
Efficiency and High Output: Automated processes ensure high-speed production with minimal human intervention.
Quality Assurance: Advanced inspection systems guarantee consistent product quality.
Regulatory Compliance: Designed to meet the strict requirements of the pharmaceutical industry.
Cost Savings: Reduces labor costs and minimizes material waste.
Flexibility: Can be easily adjusted to accommodate different products, packaging types, and production capacities.
Traceability: Serialization systems ensure full traceability throughout the supply chain.
Customization Options
Packaging formats (e.g., blister pack sizes, bottle capacities).
Output capacity and production speed.
Integration with existing manufacturing systems.
Optional features such as serialization, tamper-evident seals, or advanced vision systems.
Conclusion
The medicine packing production line is an ideal solution for pharmaceutical manufacturers looking to enhance their packaging operations. It ensures high efficiency, accuracy, and compliance with industry standards, enabling manufacturers to meet market demands while maintaining product integrity and consumer safety.